Introduction to Chromatography

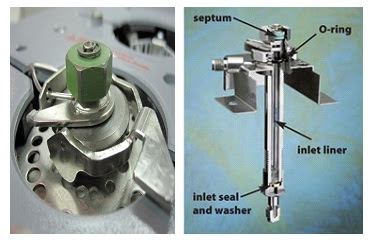
Chromatography is the science of separating mixtures from complex to simple. It is a collective term for a set of laboratory techniques as well as equipment used by scientists. Chromatography techniques are either preparative or analytical. These chromatography techniques are used by researchers and scientists throughout the world. It is indeed a need for researchers because these techniques can simplify the complex problem. Chromatography technology is used by several science-related fields such as medicine, microbiology, pharmacology, botany, chemistry, physics etc. “SCIENCE OF CHROMATOGRAPHY” will provide you with a better understanding of different chromatographic techniques, their application, mechanisms, most popular modern chromatographic techniques and so on. We welcome all our readers to gain this precise knowledge of chromatography for your relevant tasks. Topics covering... Chromatographic Techniques Common Chromatography Facts Gas Chromatography High-Performance Liquid Ch
.jpg)

















.jpg)