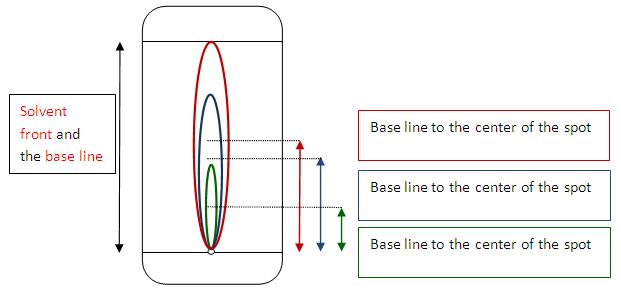
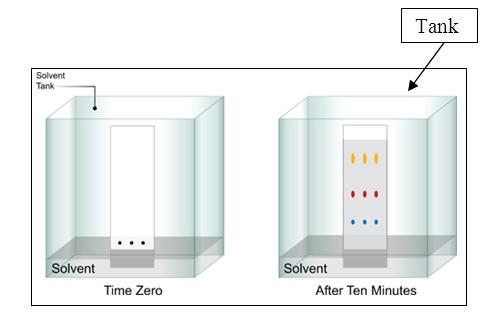
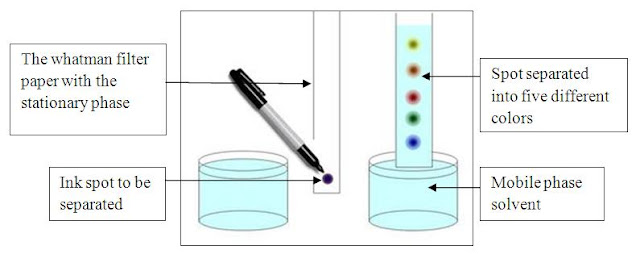
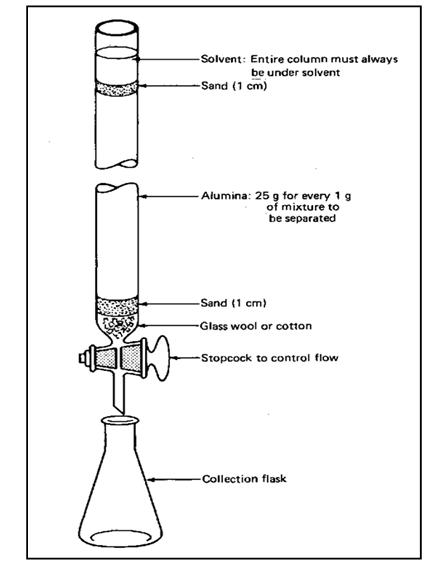
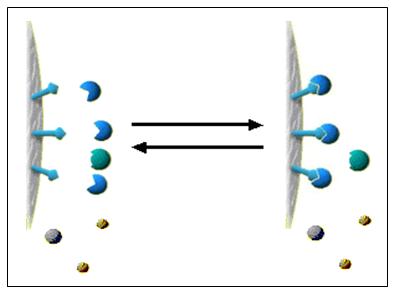
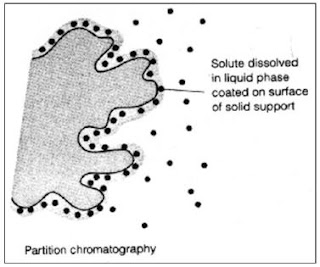
Thin Layer Chromatography (TLC)

Thin layer chromatography is another chromatographic technique used mainly to separate organic compounds. As in other methods this also has the two phases known as mobile phase and stationary phase. The mobile phase is an organic solvent. Stationary phase is SiO 2 (Silicon dioxide) applied on to an inert solid support. The inert material can be either Aluminum (Al) or a glass based tool. Both of these inert solid supports are commercially available. Inert solid support is essential because this technique is resistance to chemicals. SiO 2 is applied as thin slurry on this inert solid support to get maximum effects. In this technique very minute quantities of test sample is used. Thin layer chromatography is a highly sensitive and rapid technique. Thin layer chromatography includes the sections as following. Each section has a clear detail description. Mechanismof separation in Thin layer chromatography Procedure of Thin layer chromatography Identificationand quantifi