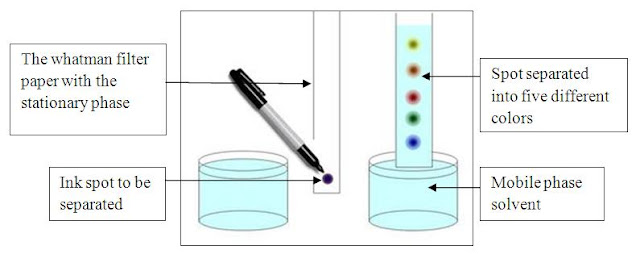
Paper Chromatography Separation Mechanisms
The
mobile phase rises up by the capillary action. The testing sample is
concentrated as a minute spot at the bottom of the filter paper. When the
mobile phase which is a liquid, rises up in the filter paper the spotted
mixture is gradually rises with the mobile phase. This eventually leads to the
separation of the compounds. Compounds in the mixture will be separated
according to their ability of the solubility. In other words it is again the
polarity as in open column chromatography. More
polar substances will move slower and less polar substances will travel faster.
Consider “A” substance is more soluble than “B” in the stationary phase, thus
“A” will dissolve in that solvent. This means “A” is more polar than “B” with
respect to the stationary phase solvent. Thus “A” will travel slowly than “B”.
And “B” will elute first. This explains that substances that having more
solubility in stationary phase move slower and substances having less
solubility in stationary phase move faster.
If you going to start a essay then you have to make a great study on your essay topic. In my academical essay I had taken a online essay writing service to complete my essay. Because they can write a best essay as our specifications. I kept the service link in the source. Evolution Writers Good luck!
ReplyDelete