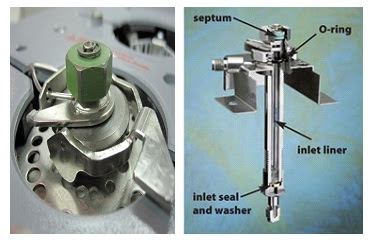
Flame Ionization Detector in Gas Chromatography (FID)
.jpg)
Flame ionization detector is the most common type of detector used in GC. In flame ionization detector Hydrogen (H2) and Oxygen (O2) are present in the gas form. This will create a flame. The components that are eluting out will burn by the flame and will turn into ions. The formation of ions will ultimately create an electrical current. Electrical current depends on the components present in the sample. The following reaction will occur in order to form the electrical current.