Parts of The Column and Its Procedure of Open Column Chromatography
This
is a glass column with two open ends. Usually in small laboratories a burette
is used instead of a glass column. The upper end should be wider than the
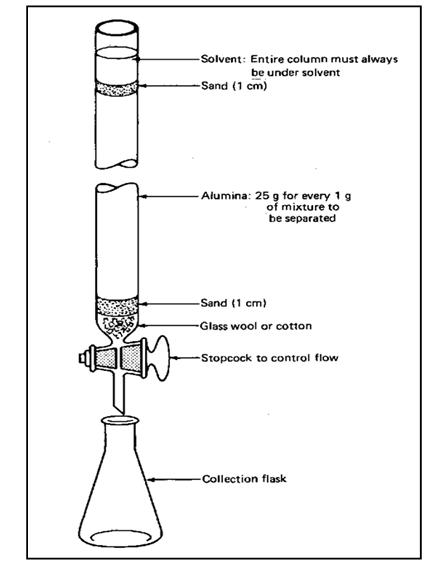
bottom end. Also at the bottom there should be a stopcock to control the
solvent flow. The bottom end is packed with glass wool or cotton wool. It is to
prevent the occurring of any damages to the stopcock from the solvent flow and
to support the stationary phase. A solid packing material is filled as the
stationary phase. The solid material can vary depending on the mode of
separation such as adsorption, partition etc.
The
mode of separation of this technique is adsorption and the separation is based
on the polarity of the substances. The stationary phase used in this is a
finely divided polar material. It is finely divided in order to increase the
surface area and it should be polar than the mobile phase that is used. Most
common packing materials are silica gel (SiO2), Alumina (Al2O3)
and also cellulose MgO and BaSO4 are also been used. The mobile
phase is a solvent or a solvent mixture. Solid adsorbent should be in uniform
size and should have a large surface area. Also the solvent (mobile phase)
should be relatively less polar than the compounds to be separated.
Nice Information!!
ReplyDeleteRead more about Column Packing